Abstract
Adult hematopoietic stem cells (HSCs) provide an attractive target for gene modification strategies for the correction of genetic blood disorders. A significant barrier to the translation of these strategies to the clinic has been the decline in numbers of fully functional HSCs associated with ex vivo culture. In order to create optimal conditions for gene modification, HSCs are typically cultured with cytokines to trigger their proliferation. Cytokine stimulation, however, induces stem cell differentiation, resulting in loss of long-term marrow repopulating potential. To overcome this barrier, we treated two sources of adult HSCs, G-CSF mobilized peripheral blood (mPB) and bone marrow (BM) CD34+ cells with valproic acid (VPA), a histone deacetylase inhibitor. This approach has previously been shown to promote the expansion of fully functional cord blood HSCs (Chaurasia et al. JCI, 2014).
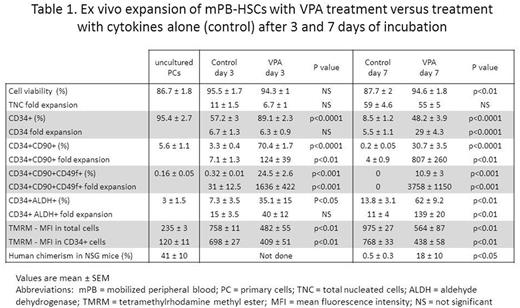
Cryopreserved mPB or BM CD34+ cells were thawed and cultured in serum-free media supplemented with stem cell factor, FLT-3 ligand, thrombopoietin and interleukin 3. The cells were primed with cytokines for 16 hours followed by addition of VPA (1mM) and incubation in the presence of VPA and cytokines for 7 days. The viability, number of total nucleated cells (TNC), CD34+ cells and frequency of various HSC subpopulations were determined shortly after thawing the primary cells (PCs) and at daily time points, in both VPA-treated cultures and cultures containing cytokines alone (control).
The viability of mPB and BM cells treated with VPA for 7 days, each exceeded 90%. While not significantly affecting the expansion of TNC, VPA treatment maintained a higher percentage of CD34+ cells throughout the culture period, and led to a higher expansion of CD34+ cells compared to that observed in control cultures (29 vs 5.5-fold in mPB and 21 vs 5-fold in BM, p<0.0001). The CD34+ fraction was further assessed for the co-expression of CD90 and CD49f, which are known to enrich for long-term repopulating HSCs. While only a small percentage of CD34+ cells from either uncultured PCs or control cultures expressed CD90 and CD49f (range 1-10%), up to 70% and 60% of VPA-treated CD34+ cells expressed CD90 and CD49f, respectively, after 24-48 hours of exposure to VPA. This rapid increase in phenotypically defined HSCs occurred prior to cell proliferation, and is attributed to epigenetic reprogramming. The combined effect of epigenetic reprogramming and proliferation led to a significant expansion of CD34+CD90+ and CD34+CD90+CD49f+ cells in both mPB and BM, not observed in the control cultures (Table 1).
Since the phenotype of cells expanded ex vivo does not always correlate with function, we used aldehyde dehydrogenase (ALDH) activity as a functional marker of HSCs. VPA-expanded mPB CD34+ cells showed significantly higher ALDH activity compared to PCs and controls, after both 3 and 7 days of incubation (Table 1). We have previously shown that epigenetic reprogramming induced by VPA is linked to mitochondrial remodeling and suppression of ROS (Papa et al. ASH 2016). We used the mitochondrial membrane potential dye TMRM to assess mitochondrial activity, and found a significant decrease in TMRM in VPA-treated cultures from mPB, as compared to control cultures, at 3 and 7 days (Table 1). This reduction in mitochondrial membrane potential is associated with primitive HSCs.
To test their functional capacity, we transplanted the progeny of 2X105 mPB and BM CD34+ cells cultured with or without VPA, into sub lethally irradiated NSG mice. The degree of human cell chimerism in mouse bone marrows was determined 16 weeks later by human CD45 expression. While mice transplanted with grafts from the control cultures had limited or no human chimerism (range 0-1.4%), those transplanted with VPA-treated mPB grafts had up to 50% human chimerism (range 2-50%) (Table 1). The engrafted cells belonged to both myeloid and lymphoid lineages. Comparable results were observed using BM.
Several groups have demonstrated the application of expansion methods adapted from cord blood to adult HSCs. These efforts met with successful albeit modest results, perhaps owing to a limited effect of the evaluated agents on adultHSCs. To our knowledge, the above studies represent the most robust demonstration of expansion of HSCs from adult sources, evident as early as after 3 days of culture. These studies strongly support the evaluation of VPA treatment in HSC gene modification strategies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.